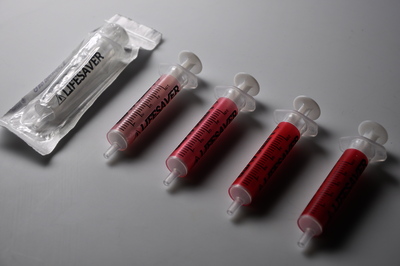
Injections are one of the most common healthcare procedures performed in the world and the most deadly. Each year 16 billion injections are delivered using a pre-used syringe. This action kills 1.3 million patients and accounts for 26-million life years lost and 32% of all new Hepatitis B cases. To prevent such violations the auto-destruct syringe was introduced in 1986. This device has since become a prerequisite tool for immunization programmes (95%) but only used for 5% of all curative injections due to excessive cost. An inclusive two-year study has developed an effective innovation strategy to contribute to a reduction of curative syringe misuse by design. Precedent case studies, force-field analysis, and dialogues with global networks and specialists has captured the complexity of the challenge, sharpened the acuity of our strategic approach and identified a need for a frugal solution that offers unilateral benefits. The outcome of the research is a transformative label technology that synthesizes theories of risk perception, chromism and visual design. Primary beneficiaries include patients, families, healthcare providers, federal government and manufacturers in both industrialised markets and developing regions- as a package sterility monitor during the supply chain or as a visual label indicating prior use for syringes or any medical device. The significance of our research is recognized by Helene Moller, Head of UNICEF’s Health Techology Centre; Peter Evans, Former Head of WHO Procurement Worldwide; Stefan Gabriel 3M’s Vice President of New Ventures and Marc Koska OBE, Founder of the SafePoint Trust. A new global mandate is being prepared by the World Health Organisation in conjunction with SafePoint that seeks to homogenise the production, safety and quality of future WHO-certified AD syringes and injection devices for safety. This award-winning innovation is included as part of this initiative.
A_Behaviour_Changing_Syringe.jpg - Submitted Version
Download (16MB) | Preview
A_Behaviour_Changing_Syringe_(2).jpg - Submitted Version
Download (1MB) | Preview
A_Behaviour_Changing_Syringe_(3).jpg - Submitted Version
Download (2MB) | Preview
A_Behaviour_Changing_Syringe_(4).jpg - Submitted Version
Download (1MB) | Preview
A_Behaviour_Changing_Syringe_(5).jpg - Submitted Version
Download (1MB) | Preview
A_Behaviour_Changing_Syringe_(6).jpg - Submitted Version
Download (2MB) | Preview
A_Behaviour_Changing_Syringe_(research).jpg - Submitted Version
Download (3MB) | Preview
A_Behaviour_Changing_Syringe_(research)_(2).jpg - Submitted Version
Download (627kB) | Preview
A_Behaviour_Changing_Syringe_(research)_(3).jpg - Submitted Version
Download (16MB) | Preview
Sterility_monitor:_prior_use_indicator_labels_(research).jpg - Submitted Version
Download (4MB) | Preview
Downloads
Downloads per month over past year
Downloads per month over past year for
"A_Behaviour_Changing_Syringe.jpg"
Downloads per month over past year for
"A_Behaviour_Changing_Syringe_(2).jpg"
Downloads per month over past year for
"A_Behaviour_Changing_Syringe_(3).jpg"
Downloads per month over past year for
"A_Behaviour_Changing_Syringe_(4).jpg"
Downloads per month over past year for
"A_Behaviour_Changing_Syringe_(5).jpg"
Downloads per month over past year for
"A_Behaviour_Changing_Syringe_(6).jpg"
Downloads per month over past year for
"A_Behaviour_Changing_Syringe_(research).jpg"
Downloads per month over past year for
"A_Behaviour_Changing_Syringe_(research)_(2).jpg"
Downloads per month over past year for
"A_Behaviour_Changing_Syringe_(research)_(3).jpg"
Downloads per month over past year for
"Sterility_monitor:_prior_use_indicator_labels_(research).jpg"
Downloads per month over past year for
"rims_2.pdf"

![Sterility monitor/ prior use indicator labels [thumbnail of Sterility monitor/ prior use indicator labels]](https://eprints.hud.ac.uk/16087/1.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe.jpg)

![Sterility monitor/ prior use indicator labels [thumbnail of Sterility monitor/ prior use indicator labels]](https://eprints.hud.ac.uk/16087/7.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe_%282%29.jpg)
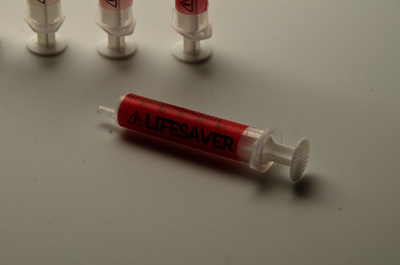
![Sterility monitor/ prior use indicator labels [thumbnail of Sterility monitor/ prior use indicator labels]](https://eprints.hud.ac.uk/16087/8.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe_%283%29.jpg)
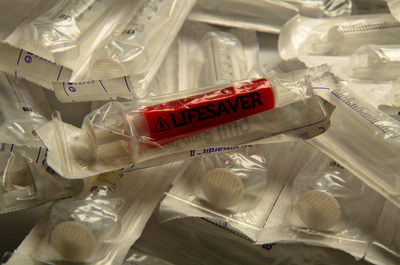
![Sterility monitor/ prior use indicator labels [thumbnail of Sterility monitor/ prior use indicator labels]](https://eprints.hud.ac.uk/16087/27.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe_%284%29.jpg)
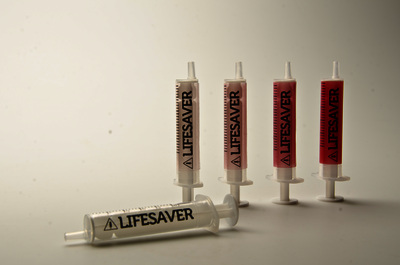
![Sterility monitor/ prior use indicator labels [thumbnail of Sterility monitor/ prior use indicator labels]](https://eprints.hud.ac.uk/16087/28.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe_%285%29.jpg)
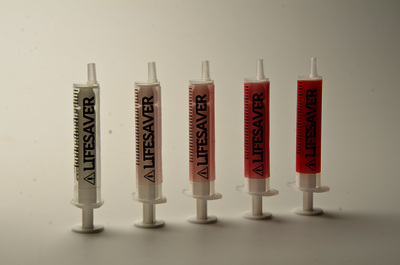
![Sterility monitor/ prior use indicator labels [thumbnail of Sterility monitor/ prior use indicator labels]](https://eprints.hud.ac.uk/16087/29.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe_%286%29.jpg)
![Sterility monitor/ prior use indicator labels (research) [thumbnail of Sterility monitor/ prior use indicator labels (research)]](https://eprints.hud.ac.uk/16087/6.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe_%28research%29.jpg)

![Sterility monitor/ prior use indicator label (research) [thumbnail of Sterility monitor/ prior use indicator label (research)]](https://eprints.hud.ac.uk/16087/9.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe_%28research%29_%282%29.jpg)

![Sterility monitor/ prior use indicator labels (research) [thumbnail of Sterility monitor/ prior use indicator labels (research)]](https://eprints.hud.ac.uk/16087/26.hassmallThumbnailVersion/A_Behaviour_Changing_Syringe_%28research%29_%283%29.jpg)

![Sterility monitor/ prior use indicator labels (research) [thumbnail of Sterility monitor/ prior use indicator labels (research)]](https://eprints.hud.ac.uk/16087/46.hassmallThumbnailVersion/Sterility_monitor%3A_prior_use_indicator_labels_%28research%29.jpg)


 CORE (COnnecting REpositories)
CORE (COnnecting REpositories) CORE (COnnecting REpositories)
CORE (COnnecting REpositories)